American doctors succeeded in performing an operation on the brain of a fetus in its mother's womb, to treat a rare medical condition, which is the first in the world.
Surgeons at Boston Children's Hospital and Brigham's Hospital in Massachusetts used a surgical technique to treat a congenital malformation known as a "Gallen vein malformation," which causes heart failure and stroke-like symptoms within days of birth.
Using ultrasound, doctors were able to treat the deformity, which causes blood to flow too quickly through part of the brain, the first such condition to be treated in this way.
Darren Orbach, a neuroradiologist at Boston Children's Hospital and Harvard Medical School, said that after the operation and birth, the infant did not suffer from blood flow as it usually happens to those with deformity.
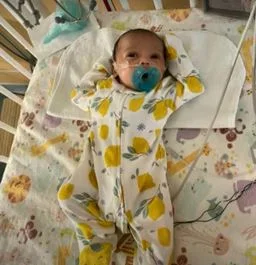
Auerbach and his colleagues performed the procedure on a fetus at 34 weeks' gestation, and used ultrasound to guide them through the procedure. And the girl was born after two days, because the operation leads to the rupture of the membranes in the uterus, and the child is born prematurely.
Because the birth was premature, the infant was transferred to the hospital's neonatal intensive care unit for several weeks, during which time doctors continued to monitor her brain.
Orbach revealed that the infant is now in the sixth week, does not take any medications, eats normally and gains weight, and does not suffer any negative effects on her brain.
Doctors confirmed that the infant girl was monitored in the neonatal intensive care unit for several weeks before she was discharged from the hospital to the home, explaining that during this period, the infant underwent a normal neurological examination and no clots, fluid accumulation or bleeding appeared in the MRI scans of the brain.
The US FDA warns of the danger of products that promise to "increase energy" : Live Science
The US Food and Drug Administration (FDA) has warned that many unapproved inhalers marketed as "energy boosters" often contain ammonia, a toxic gas that can cause several symptoms.
The FDA warning reads: “Inhalation of ammonia can quickly lead to eye, nose, and throat irritation, coughing, and airway constriction. The FDA has received reports of adverse events such as shortness of breath, seizures, migraines, vomiting, diarrhea, and fainting from consumers who used non-steroidal stimulants. approved.
The US Food and Drug Administration (FDA) issued its consumer safety alert after sending a letter to the product's manufacturer, Nose Slap LLC, on April 24. The warning message mentions the two alarms that are sold on the company's website.
According to the letter, Nose Slap and Soul Slap are advertised as energy-boosting sugar and caffeine alternatives, and the company's website notes that both inhalants contain ammonia.
Specifically, the products are described as salts with an "extremely strong" smell. In general, olfactory salts using ammonia irritate the mucous membranes of the upper respiratory tract and stimulate the sniffing reflex. This reflex, in turn, alters a person's breathing and increases oxygen flow and gas exchange in their lungs, which may increase alertness.
Once used to prevent or treat fainting, aroma salts are no longer used regularly by doctors, but are sometimes used by athletes in an attempt to improve performance although there is little evidence to support this use. However, odor salts are relatively safe - but inhaling ammonia frequently or in high doses is harmful. Again, because Nose Slap and Soul Slap are not approved, the FDA cannot guarantee the quality or safety of the products.
Depending on the inhaled dose, ammonia can burn the tissues of the nose, throat, and trachea; It causes swelling and fluid buildup in the lungs. It causes "airway destruction leading to respiratory distress or failure," in addition to the symptoms listed earlier, according to the Centers for Disease Control and Prevention (CDC).
The CDC notes that those who survive breathing very high concentrations of ammonia can suffer long-term lung damage, and if the chemical gets into the eye, it can damage the cornea and sometimes lead to blindness.
In its warning statement, the FDA encouraged healthcare professionals and consumers to report adverse events or quality problems with the Nose Slap or Soul Slap to the MedWatch Adverse Event Reporting Program.
The FDA stated: “These products have not been demonstrated to be safe or effective for their intended uses. Failure of the Company to promptly correct violations may result in legal action without further notice, including, but not limited to, product seizure and warrant judicial".
Tags:
health